26 Mai 2023
The ACCESS project, whose name is an acronym standing for Advanced Cell Control by Spectroscopic Sensors, is an ambitious initiative aiming to transform the production of Advanced Therapy Medicinal Products (ATMP) by making these processes more efficient and less costly. This project is part of a response to the societal challenges linked to the production of therapeutic cells, particularly Mesenchymal Stem Cells (MSCs), used in various medical applications, including tissue regeneration.
Technological Objectives and Innovations
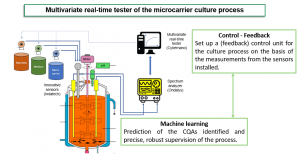
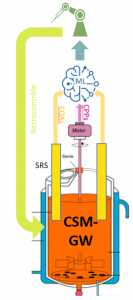
The main goal of the ACCESS Project is to optimize MSC culture in bioreactors through an integrated approach combining innovative optical sensors, machine learning algorithms, automatic real-time feedback and advanced modeling of the process by means of a digital twin. The methodological framework is based on the Quality-by-Design (QbD) approach, with the aim of ensuring product quality from the design phase onwards by integrating in-line inspections at each stage of the manufacturing process.
More specifically, the project is focusing on:
- Development of clinical-grade bioreactor culture processes.
- Design and validation of innovative optical sensors for critical quality attributes (CQAs) in real time.
- Integration of machine learning techniques to merge the data collected and continuously supervise the process.
- Implementation of an automated feedback system to adjust the process parameters in real time.
- Modeling by means of a digital twin, making it possible to simulate and optimize the production processes.
Indatech’s Involvement
One of the key partners in the ACCESS consortium is INDATECH, a Chauvin Arnoux Group company specialized in the design and manufacture of advanced optical sensors. INDATECH plays a crucial role in the implementation of sensors for inspecting cell cultures in-line. These sensors, which are essential for accurate real-time monitoring of CQAs such as cell density and the degree of microcarrier coverage, lie at the heart of the system allowing automation of the feedback process. Thanks to these innovations, INDATECH contributes significantly to production cost reductions while improving reliability and ATMP quality.
In collaboration with other partners such as Ondalys, Cybernano, Ypso Facto, MTInov and StemInov, INDATECH is helping to structure an integrated French QbD/PAT offering for the whole of Europe. This offering will cover all the adherent cell culture inspection requirements in the biotechnology sector, from modeling of the processes to industrial implementation of in-line inspection technologies.
Socio-economic Impact and Outlook
The ACCESS project is not limited to technological developments. It also expects to see significant economic fallout, notably with the creation of around ten jobs in R&D, in design offices and in services. In addition, it will strengthen two strategic industrial poles: one for the production of clinical-grade MSCs, and the other for designing and manufacturing optical sensors, thus helping to create jobs and expand the infrastructures.
In terms of intellectual property, at least two patents are expected to be filed, bearing witness to the level of innovation involved in this project.
Thanks to synergy between the skills of the partners involved and the technological innovation contributed by INDATECH, the ACCESS project represents a major step forward in the production of Advanced Therapy Medicinal Products. By optimizing the stem cell culture processes, this project addresses the growing requirements of regenerative medicine while encouraging the emergence of a French industrial sector which is competitive at European level.